MyPeriodic Table
- Invest in your education… invest in your future.
- Makes a great gift for science students and enthusiasts.
- Back to school special!
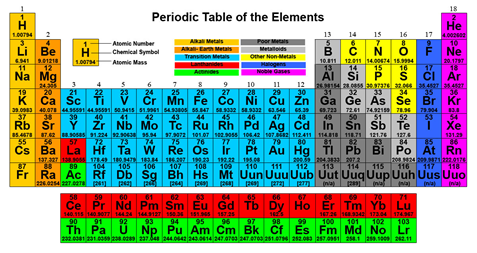
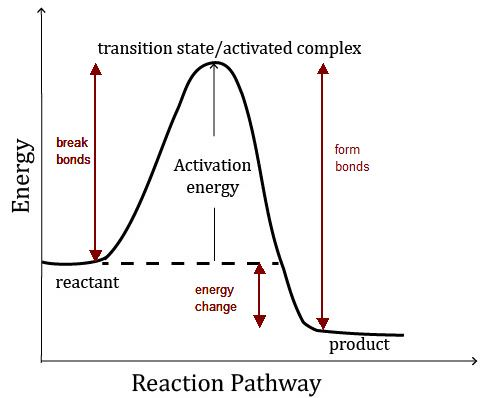
- Chemistry is a very broad field of study, and for many or most, very frightening. The intention of this work is not to replace quality text books or formal education. We designed this product to be a refresher and reference to the student, novice and practicing professional. This was originally built off of the Periodic Table. The Periodic Table is likely to be the most recognizable symbol of chemistry and also it's single most useful tool.
-
Bullets
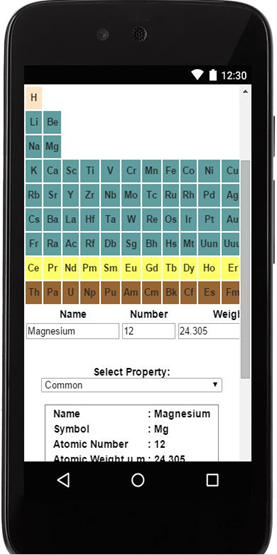
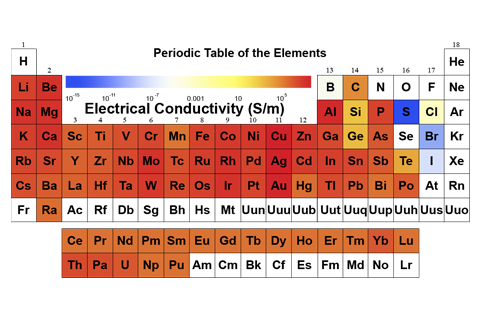
- Comprehensive database on elemental data (see data list). Select data by either choosing element or data family… one touch.
- Database uses asynchronous javascript. All operations are resident, no web searching or roaming required.
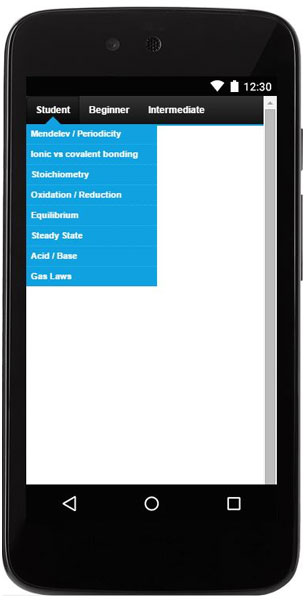
- Diverse subject area for a wide variety of interests and experience levels.
- Completely self contained. Only external operation is if user desires to send an email to us.
- Developed by chemistry professionals, chemistry professors, pharmacists and other science professionals.
- No advertising.
- Clean, simple interface design
-
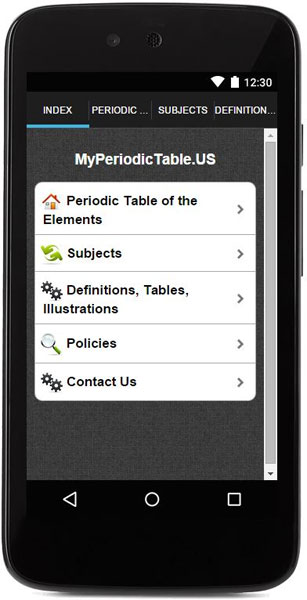
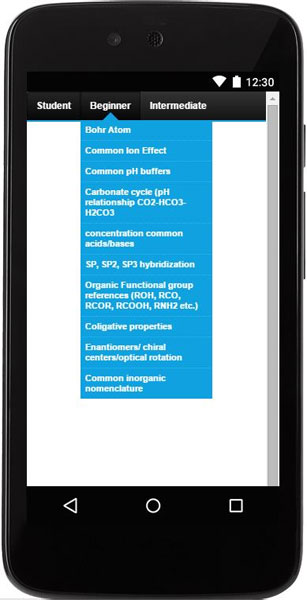
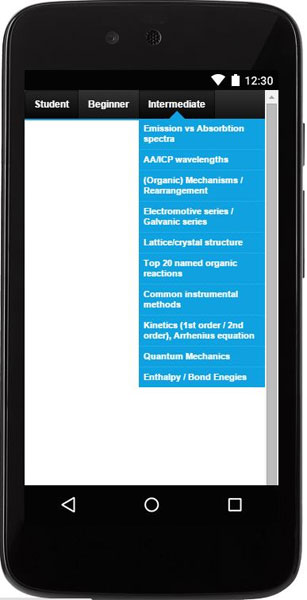
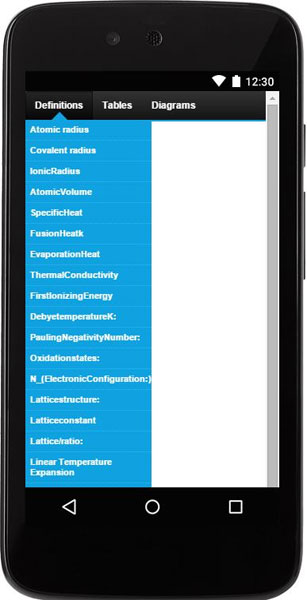
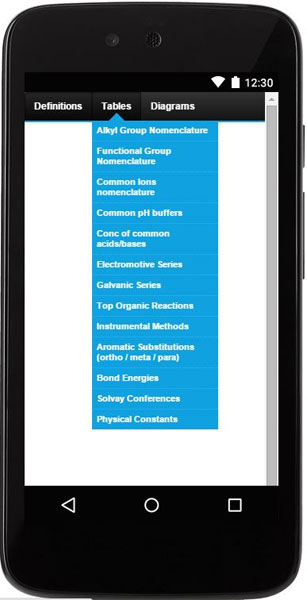
This products consists of three useful tools:
- Periodic Table and javascript database.
- Subjects list (28 topics).
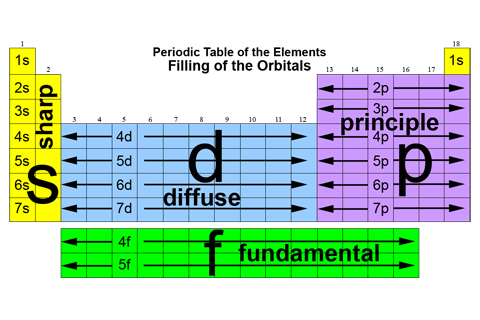
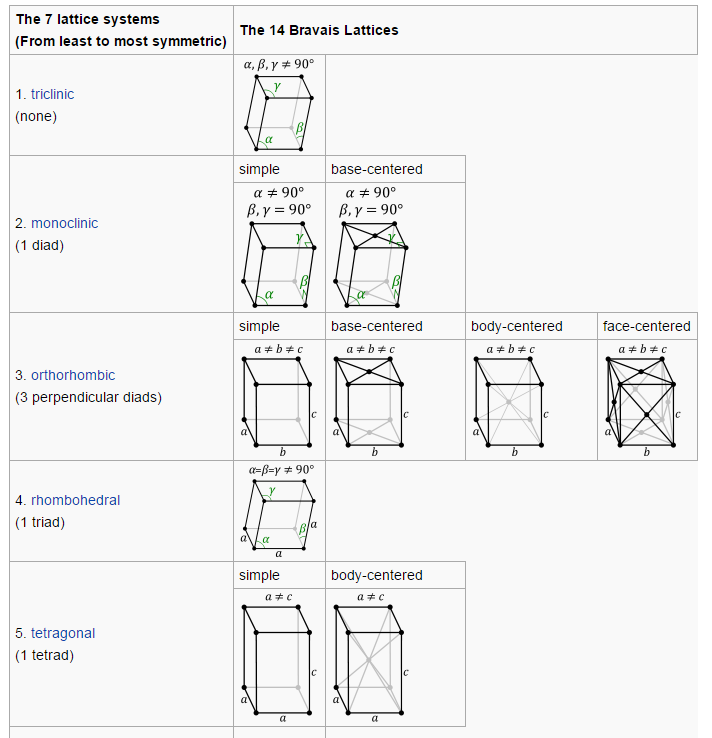
- Definitions, tables and illustrations (for easy look up).